How Important Is Regulatory Compliance in Menstrual Pain Patch Production?
Ensuring Safety, Trust, and Global Market Access through Compliance Standards
Introduction: Why Regulatory Compliance Defines Success in Menstrual Pain Relief Patch Manufacturing
In the rapidly expanding feminine wellness industry, menstrual pain relief patches have gained significant popularity as a non-invasive, convenient, and natural solution to menstrual discomfort. However, behind every successful product lies a crucial element that determines both its credibility and market success — regulatory compliance.
Whether you are working with a Menstrual Pain Relief Patches Manufacturer, collaborating with a Menstrual Pain Relief Patches OEM, or launching Custom Menstrual Pain Relief Patches under your own brand, compliance with local and international standards is non-negotiable. It ensures product safety, maintains consumer trust, and opens the door to global markets.
This article explores the vital role of regulatory compliance in menstrual pain patch production and how small and large brands can work with reliable Menstrual Pain Relief Patches Suppliers to achieve consistent quality and market success.
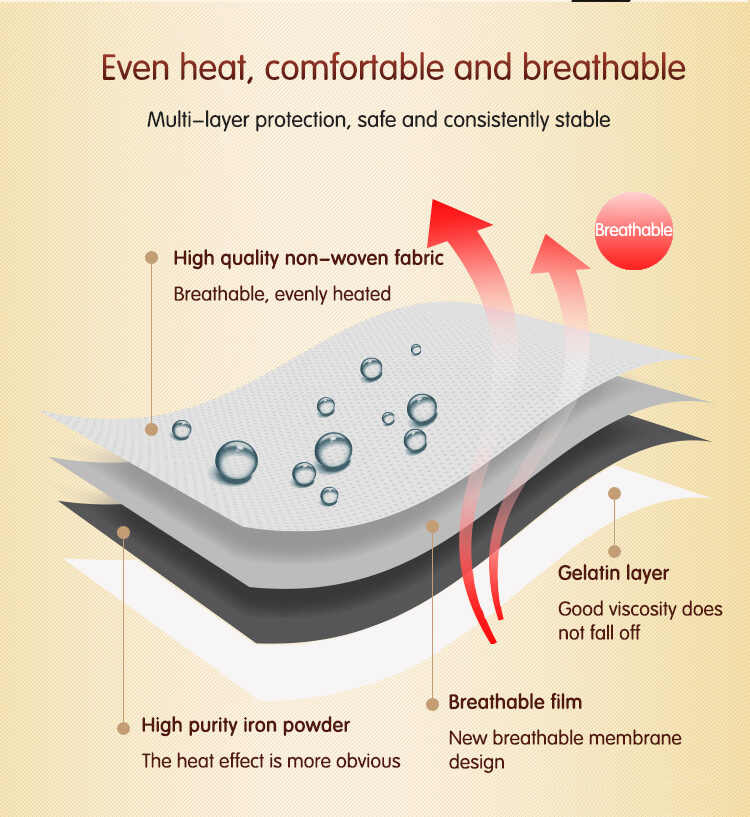
1. What Does Regulatory Compliance Mean in Menstrual Pain Patch Manufacturing?
Regulatory compliance refers to adhering to the laws, standards, and guidelines that govern the manufacturing, labeling, and distribution of healthcare and cosmetic products. For Menstrual Pain Relief Patches Manufacturers, this includes meeting safety, efficacy, and hygiene standards set by international regulatory authorities such as:
FDA (U.S. Food and Drug Administration) – For ensuring product safety and proper labeling in the U.S.
CE Marking (Conformité Européenne) – Required for products sold in the European Union.
ISO and GMP Certifications – Indicating that manufacturing processes meet global standards of quality and safety.
A compliant Menstrual Pain Relief Patches OEM ensures that every step — from ingredient selection to final packaging — meets these requirements, minimizing risks and ensuring consistent performance.
2. Why Regulatory Compliance Is Essential in Menstrual Pain Patch Production
a. Ensures Consumer Safety
The primary reason for strict compliance is consumer safety. Menstrual patches are applied directly to the skin, often for extended periods. Poor-quality adhesives or untested ingredients can cause allergic reactions, irritation, or burns.
A certified Menstrual Pain Relief Patches Manufacturer uses dermatologically tested materials, non-toxic adhesives, and skin-friendly formulations to prevent these risks. Compliance ensures the product is safe for long-term use and suitable for sensitive skin.
b. Builds Brand Credibility and Consumer Trust
Today’s consumers are more informed than ever. They actively look for certifications and safety assurances before making a purchase. A brand that promotes FDA-registered or CE-certified Private Label Menstrual Pain Relief Patches instantly gains more credibility.
Regulatory compliance signals professionalism, responsibility, and reliability — values that drive long-term customer loyalty and word-of-mouth marketing.
c. Reduces Legal and Financial Risks
Failing to comply with industry standards can result in product recalls, lawsuits, or regulatory bans. Partnering with a compliant Menstrual Pain Relief Patches OEM protects your business from costly legal complications and ensures smooth market operations.
d. Facilitates Global Market Expansion
Regulations vary by country. Compliance with international standards such as ISO 13485 (medical devices) and GMP enables your Custom Menstrual Pain Relief Patches to enter multiple markets without additional certification hurdles. This is especially valuable for brands aiming to expand into Europe, the U.S., or Asia-Pacific regions.
3. Key Areas of Regulatory Compliance in Menstrual Pain Patch Production
a. Ingredient Safety and Quality Control
Every raw material — from herbal extracts to adhesives — must undergo strict quality testing. Reputable Menstrual Pain Relief Patches Suppliers follow GMP guidelines to ensure each ingredient is pure, consistent, and free from contaminants.
Herbal or active ingredients should also be validated for their therapeutic claims to prevent misleading advertising. For instance, if a brand claims “fast pain relief,” that must be supported by clinical or lab data.
b. Manufacturing Process Standards
A Menstrual Pain Relief Patches Manufacturer must maintain a cleanroom environment, calibrated machinery, and trained staff to ensure product uniformity. ISO and GMP certifications validate these practices.
Compliance also involves documentation of every step — from batch records to quality inspections — to ensure full traceability and accountability.
c. Product Testing and Stability Evaluation
Before market release, Custom Menstrual Pain Relief Patches must undergo various safety and performance tests:
Dermatological Testing – Ensures the patch is safe for skin contact.
Microbiological Testing – Confirms the absence of harmful microorganisms.
Stability Testing – Assesses shelf life under different environmental conditions.
Efficacy Testing – Validates pain relief performance over time.
d. Labeling and Packaging Compliance
Regulations also govern what information must appear on product packaging — including ingredients, usage instructions, warnings, and shelf life. Mislabeling or false claims can lead to serious regulatory actions.
A reliable Menstrual Pain Relief Patches OEM ensures your labels meet local and international packaging laws, reducing risks and increasing consumer transparency.
4. The Role of OEMs in Maintaining Regulatory Compliance
For brands entering the menstrual wellness market, working with a professional Menstrual Pain Relief Patches OEM offers a major advantage — the OEM takes on much of the compliance responsibility.
Here’s how:
Documentation Support: OEMs maintain detailed manufacturing and testing records for regulatory audits.
Certification Assistance: They provide ISO, GMP, and CE documentation for product registration.
Testing Coordination: OEMs handle third-party safety and stability testing.
Regulatory Updates: Reputable manufacturers keep track of evolving laws to ensure continued compliance.
This partnership allows small and medium-sized brands to focus on marketing and distribution, knowing their products meet all required safety standards.
5. The Impact of Non-Compliance on Brands
Ignoring or underestimating compliance requirements can have severe consequences for both Private Label Menstrual Pain Relief Patches and Custom Menstrual Pain Relief Patches brands.
a. Product Recalls and Market Bans
Non-compliant products can be removed from the market, resulting in financial loss, damaged reputation, and lost consumer trust.
b. Legal Liabilities
If a product causes harm, even unintentionally, the brand can face lawsuits, fines, and potential criminal charges.
c. Loss of Business Partnerships
Retailers and distributors prefer to work with certified Menstrual Pain Relief Patches Suppliers who can guarantee compliance. Non-compliance limits your business opportunities and market reach.
6. How to Verify an OEM’s Compliance Standards
Before partnering with a Menstrual Pain Relief Patches Manufacturer, brands should take these steps to confirm compliance:
Request Certificates – Verify ISO, GMP, FDA, or CE certifications.
Audit the Facility – If possible, conduct an on-site or virtual audit.
Ask for Documentation – Review testing reports and quality control records.
Evaluate Communication Transparency – A compliant manufacturer will openly share compliance-related details.
Check References – Contact previous clients to assess reliability and performance.
Choosing a compliant Menstrual Pain Relief Patches OEM not only ensures safety but also strengthens your brand’s reputation in global markets.
7. Compliance and Innovation: A Balanced Approach
While compliance is mandatory, it should not hinder innovation. Leading Menstrual Pain Relief Patches Manufacturers combine strict regulatory adherence with advanced research to develop new and improved patch technologies.
For example:
Biodegradable materials to meet environmental standards.
Enhanced heat distribution layers for better pain relief.
Natural herbal blends supported by clinical studies.
Such innovations not only maintain compliance but also attract eco-conscious and health-focused consumers.
8. The Future of Compliance in the Menstrual Care Industry
As the global feminine wellness market evolves, regulatory expectations are becoming more stringent. Governments are demanding higher transparency regarding ingredient sourcing, environmental impact, and manufacturing ethics.
Forward-thinking Menstrual Pain Relief Patches Suppliers are now investing in:
Sustainable production practices.
Ethical ingredient sourcing.
Advanced traceability systems (e.g., QR code-based product verification).
Brands that partner with such responsible OEMs position themselves as trustworthy and future-ready players in the market.
Conclusion: Compliance as the Foundation of Long-Term Success
Regulatory compliance is not merely a legal requirement — it’s the foundation of consumer trust, brand reputation, and global competitiveness.
Whether you’re producing Custom Menstrual Pain Relief Patches or launching Private Label Menstrual Pain Relief Patches, aligning with a compliant and certified Menstrual Pain Relief Patches Manufacturer ensures that your products are safe, effective, and globally marketable.
In a marketplace where consumers value transparency and safety, regulatory compliance is not an obstacle — it’s your strongest competitive advantage.
Related Questions and Answers
Q1: What certifications should a Menstrual Pain Relief Patches Manufacturer have?
A1: Look for ISO 13485, GMP, CE, and FDA registrations. These indicate strong quality control and international compliance.
Q2: How do OEMs help with compliance?
A2: A Menstrual Pain Relief Patches OEM manages documentation, testing, and regulatory updates, ensuring your product meets regional and global requirements.
Q3: Are herbal ingredients in patches regulated?
A3: Yes. Even natural or herbal components must pass safety and stability tests to ensure they are non-toxic and effective.
Q4: Can non-compliance affect private label brands?
A4: Absolutely. Non-compliance can lead to recalls, fines, and permanent damage to a Private Label Menstrual Pain Relief Patches brand’s reputation.
Q5: How can I verify my supplier’s compliance?
A5: Request up-to-date certifications, review quality reports, and confirm third-party testing results before entering into any agreement.






