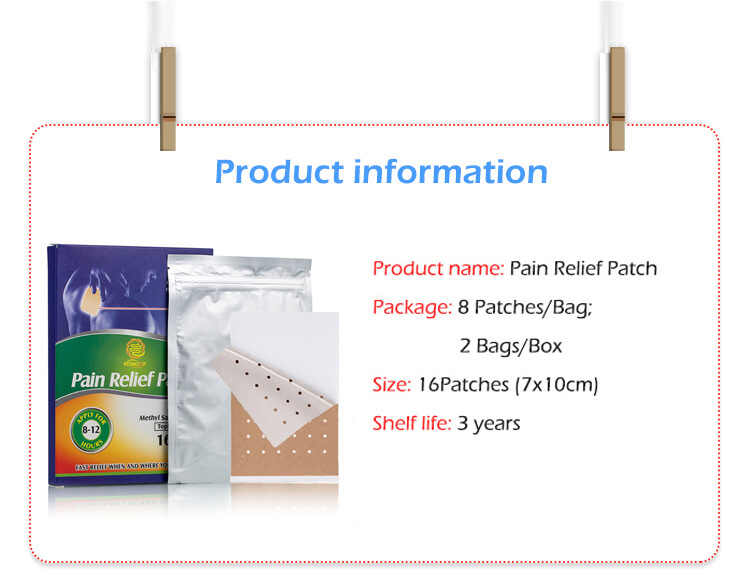
How Can I Verify if an Effective Pain Relief Patches Supplier Is GMP-Certified?
As the global demand for pain management solutions continues to grow, so does the need for trustworthy partners in the health and wellness supply chain. Whether you're working with a Private Label Effective Pain Relief Patches Manufacturer, sourcing from an Effective Pain Relief Patches OEM, or developing Custom Effective Pain Relief Patches, ensuring your Effective Pain Relief Patches Supplier is GMP-certified is crucial.
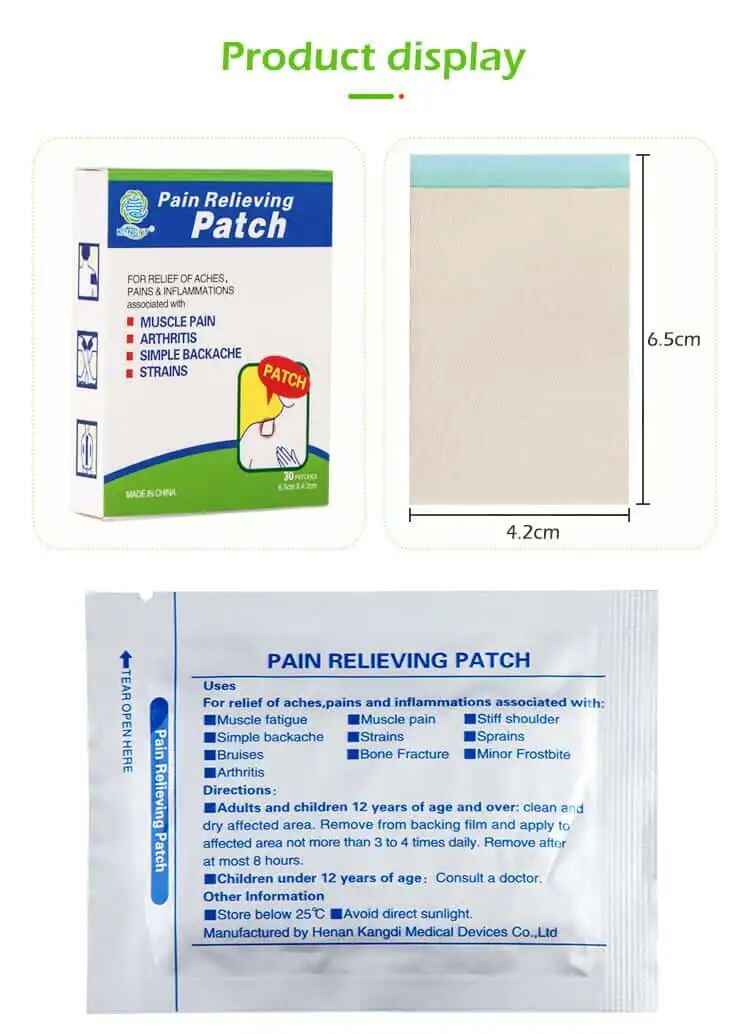
In this in-depth guide, we’ll explain:
What GMP certification means,
Why it's important,
How to verify GMP compliance,
And the implications of working with non-certified suppliers.
This guide will help you make informed decisions and protect your brand’s credibility, consumer trust, and long-term success.
What Is GMP Certification?
GMP (Good Manufacturing Practice) refers to a system that ensures products are consistently produced and controlled according to quality standards. It is designed to minimize risks involved in pharmaceutical production that cannot be eliminated through testing the final product.
A GMP-certified Effective Pain Relief Patches Manufacturer or OEM complies with stringent guidelines set by regulatory agencies such as:
The U.S. Food and Drug Administration (FDA),
The European Medicines Agency (EMA),
The World Health Organization (WHO),
And other local health authorities.
These standards cover every aspect of production—from raw materials, facilities, and equipment to training and personal hygiene of staff.
Why GMP Certification Matters for Effective Pain Relief Patches
Choosing a GMP-certified Effective Pain Relief Patches Supplier ensures:
Product Safety and Efficacy
Only certified suppliers can guarantee that the Custom Effective Pain Relief Patches you offer are safe, clean, and effective for consumers.Regulatory Compliance
Selling pain relief products in global markets often requires proof of GMP compliance. A supplier lacking this certification could prevent your product from being legally sold.Brand Credibility
A GMP certificate reinforces your position as a serious, trustworthy brand—especially when working with Private Label Effective Pain Relief Patches.Reduced Risk of Recalls
Recalls can destroy reputation and profit. Working with a GMP-compliant Effective Pain Relief Patches OEM significantly reduces that risk.
How to Verify GMP Certification of an Effective Pain Relief Patches Supplier
1. Request a Valid GMP Certificate
The simplest and most direct method is to ask the supplier to provide their GMP certificate. Ensure:
It’s issued by a recognized health authority.
It includes the name of the Effective Pain Relief Patches Manufacturer or OEM.
The certificate is current and has not expired.
Red flag: Suppliers who hesitate or refuse to share GMP documentation may not be compliant.
2. Verify Through Regulatory Bodies
Many national and international regulatory bodies offer online databases or verification services. Depending on the country of origin of your Effective Pain Relief Patches Supplier, you can check:
FDA (USA): Search the Drug Establishments Current Registration Site.
EMA (EU): Use the EudraGMDP database for EU-based manufacturers.
China NMPA: Access GMP information for Chinese Effective Pain Relief Patches OEMs.
Health Canada: Lists licensed establishments online.
Tip: Match the business name and address listed on the certificate with the listing on these databases.
3. Request a Third-Party Audit Report
If you want deeper assurance, ask the supplier for a recent third-party GMP audit report from a certified agency (e.g., SGS, Intertek, TÜV SÜD). These reports often include:
Manufacturing procedures,
Storage and sanitation practices,
Equipment calibration and maintenance records,
Staff qualifications and hygiene.
4. Visit the Facility (if feasible)
If you’re investing heavily in Custom Effective Pain Relief Patches, conducting an on-site audit is the most thorough verification method. During your visit:
Evaluate cleanliness and workflow,
Check the presence of SOPs (Standard Operating Procedures),
Interview the quality assurance team.
This is particularly important for Private Label Effective Pain Relief Patches where your brand name is at stake.
5. Use a Sourcing Agent or Consultant
Professional sourcing agents can verify the GMP status of an Effective Pain Relief Patches Supplier on your behalf. This option is great for overseas suppliers and includes:
Physical inspections,
Document verification,
Background checks.
Red Flags to Watch Out For
No expiration date on the certificate
Legitimate GMP certificates are time-bound.Mismatch in name/address
If the certificate details don’t match the supplier’s business information, that’s a problem.No third-party validation
A certificate without a traceable issuing authority should not be trusted.Unwillingness to share documentation
Transparency is non-negotiable for trustworthy partners.
What if a Supplier Isn’t GMP-Certified?
Choosing a non-GMP Effective Pain Relief Patches Manufacturer or OEM comes with serious risks:
Quality issues that could result in customer complaints or health hazards,
Legal penalties in regulated markets like the U.S., EU, or Canada,
Reputational damage that’s hard to recover from,
Higher likelihood of contamination or inaccurate ingredient labeling.
If a supplier is not currently GMP-certified but is in the process of obtaining it, evaluate their timeline and consider using them only after certification is complete.
Working with GMP-Certified OEMs for Custom Formulations
Many Effective Pain Relief Patches OEMs that offer GMP-compliant services can also assist in developing Custom Effective Pain Relief Patches. This includes:
Unique ingredient blends (menthol, camphor, CBD, herbal extracts),
Time-release or hydrogel technologies,
Skin sensitivity testing,
Packaging that complies with global standards.
Working with a GMP-certified OEM ensures that even these custom formulations adhere to stringent safety and efficacy standards.
Private Label Products and GMP: Why It Still Matters
Even if you're not formulating from scratch, GMP certification is still essential for Private Label Effective Pain Relief Patches. Why?
Your brand name appears on the product,
You are liable for product performance and safety,
Consumers expect high standards—even for white-label goods.
Thus, never skip GMP verification—even for stock formulas.
How GMP Affects Your Market Expansion
Many retailers, e-commerce platforms, and international distributors now require proof of GMP compliance before listing products. If your Effective Pain Relief Patches Supplier is not certified, you may:
Be barred from Amazon, Walmart Marketplace, or EU online pharmacies,
Struggle with customs clearance,
Face difficulty in securing partnerships or funding.
Investing in GMP-compliant suppliers pays off with easier scaling, stronger partnerships, and long-term sustainability.
Final Thoughts
Verifying if your Effective Pain Relief Patches Supplier is GMP-certified is not just a regulatory formality—it’s a critical part of building a reputable, trustworthy brand. Whether you’re working with a Private Label Effective Pain Relief Patches Manufacturer or developing a new product with a Custom Effective Pain Relief Patches OEM, always demand proof of compliance and perform your due diligence.
By choosing a certified, transparent partner, you not only protect your business legally—you also strengthen your position in a competitive and highly regulated marketplace.
Related Questions and Answers
Q1: Can I sell pain relief patches in the U.S. without GMP certification?
A1: No. The FDA requires that OTC drug products, including pain relief patches, be manufactured under GMP conditions.
Q2: How often do GMP certifications need to be renewed?
A2: Typically every 1 to 3 years, depending on the issuing authority and regulatory changes.
Q3: Do all Effective Pain Relief Patches Manufacturers have GMP certification?
A3: No. You must verify each supplier individually, especially when sourcing internationally.
Q4: Is there a difference in GMP standards across countries?
A4: Yes, but most align with WHO or ICH guidelines. Always verify that certification meets your market's regulations.
Q5: Does GMP certification apply to packaging too?
A5: Yes. Packaging must meet hygiene and labeling standards as part of GMP compliance.