How Can I Be Sure My Private Label Sports Pain Patches Are Compliant? A Complete Regulatory Checklist for Brand Owners
The U.S. sports medicine market continues to expand, and Private Label Sports Pain Patches have become an increasingly popular product offering. Whether you're a startup brand or an established retailer, ensuring that your product complies with regulatory standards is not just good business—it's essential for legal protection and long-term success.
This in-depth guide walks you through everything you need to know to ensure your Private Label Sports Pain Patches are compliant with U.S. regulations. We'll explore what Sports Pain Patches Manufacturers, Sports Pain Patches OEMs, and Suppliers must deliver, and what brand owners need to verify before going to market with Custom Sports Pain Patches.

1. Understanding Compliance: What Does It Mean for Private Label Sports Pain Patches?
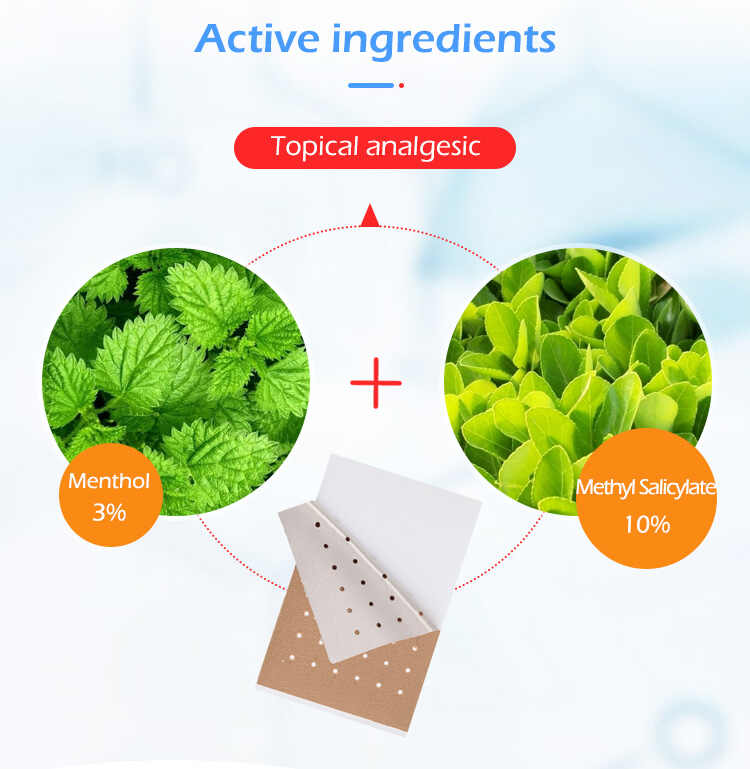
Compliance refers to following all applicable federal and state laws that govern how your product is manufactured, labeled, marketed, and sold. In the U.S., most Sports Pain Patches are regulated by the Food and Drug Administration (FDA) as Over-the-Counter (OTC) drugs, especially if they contain active ingredients like menthol, camphor, or methyl salicylate.
For a Private Label Sports Pain Patch, you are legally responsible for ensuring that your branding and product meet all regulatory requirements—even if a third-party Sports Pain Patches Manufacturer or OEM produces the product.
2. Confirm the Product Follows an FDA OTC Monograph
The FDA Monograph System provides a “pre-approved” formula and labeling standard for common OTC drug categories, including topical analgesics.
Key Ingredients Permitted Under the Monograph:
Menthol (up to 16%)
Camphor (up to 11%)
Methyl Salicylate (up to 30%)
Actions to Take:
Confirm that your Sports Pain Patches Supplier uses only monograph-compliant active ingredients and concentrations.
Ensure the formulation hasn’t been altered with unapproved or novel ingredients like CBD or high-dose lidocaine (over 4%).
Why It Matters: If your formula deviates from the monograph, you may need a New Drug Application (NDA)—a costly and time-consuming process not typically suitable for private label brands.
3. Verify Manufacturer Registration and FDA Compliance
Any facility that manufactures, packages, or labels Private Label Sports Pain Patches must be registered with the FDA.
What to Ask Your Sports Pain Patches Manufacturer or OEM:
Is your facility registered with the FDA? (Ask for their Establishment Registration Number)
Are your operations GMP compliant under 21 CFR Part 211?
Can you provide recent third-party audit reports or FDA inspection records?
Custom Sports Pain Patches produced in unregistered or non-compliant facilities risk being rejected by retailers, banned from online platforms, or subject to regulatory enforcement.
4. Ensure Proper Labeling According to FDA Guidelines
Labeling is one of the most common compliance pitfalls for Private Label Sports Pain Patches. The FDA requires a standardized Drug Facts Panel on OTC drugs.
Required Label Elements:
Active Ingredients
Purpose (e.g., topical analgesic)
Uses (e.g., temporary relief of muscle and joint pain)
Warnings (e.g., “For external use only”)
Directions
Inactive Ingredients
Contact Information for Adverse Events
Make sure your Sports Pain Patches Supplier uses the correct format and language. Claims such as “FDA Approved,” “Instant Cure,” or “Guaranteed Results” are prohibited unless backed by scientific evidence or FDA approvals.
5. Request the National Drug Code (NDC) Listing
Even if your Private Label brand doesn’t manufacture the product, your Sports Pain Patches OEM or manufacturer should list each product with the FDA’s National Drug Code (NDC) directory.
How to Check:
Ask for the NDC number assigned to your patch.
Use the FDA NDC Directory to verify.
Why It Matters: Products not listed with the FDA may be flagged by online marketplaces like Amazon or eBay and rejected by major retailers.
6. Confirm GMP (Good Manufacturing Practices) Compliance
GMP compliance ensures your product is manufactured in a clean, controlled, and quality-monitored environment.
GMP Key Areas Include:
Batch production records
Stability testing
Sanitation protocols
Proper documentation and labeling controls
Personnel training
Partner only with Sports Pain Patches Manufacturers that are cGMP certified and ideally have passed recent third-party audits or ISO certifications.
7. Confirm Product Testing and Quality Assurance
To protect your brand and consumers, confirm that your Private Label Sports Pain Patches undergo regular testing.
Key Testing to Require:
Microbial contamination
Heavy metals (especially for herbal-based patches)
Adhesive strength and release
Shelf life and stability
Ingredient verification (active and inactive)
Sports Pain Patches Suppliers should provide Certificates of Analysis (COAs) for each production batch.
8. Understand Marketing Restrictions
Even if your product is fully compliant in formulation and labeling, making inappropriate marketing claims can still violate FDA rules.
Avoid These Claims:
“Heals injuries overnight”
“Instant pain relief for all conditions”
“Safe for pregnant women” (unless clinically proven and approved)
Stick to claims that are supported by the FDA monograph, and avoid making disease treatment claims unless your product is NDA-approved.
9. Ensure State-Level and E-Commerce Platform Compliance
Some additional areas of concern:
California Prop 65 (for certain chemicals)
Amazon, Walmart, and CVS platform rules (require NDC listing, GMP proof, and product images)
Packaging material regulations in states like New York and California
Before launching, make sure your Custom Sports Pain Patches meet both federal and state-level standards.
10. Build a Compliance File for Your Brand
Create a centralized file that includes:
Product NDC listing
Manufacturer’s FDA registration info
GMP certificates or audit reports
Certificate of Analysis (COA)
Sample product labels
Marketing review checklist
This file will help you respond quickly to distributor, retailer, or regulatory inquiries and prove that your Private Label Sports Pain Patches are fully compliant.
Conclusion: Compliance Is a Competitive Advantage
Ensuring regulatory compliance isn’t just about avoiding trouble—it’s about building a sustainable brand that customers and retailers trust. By partnering with an experienced Sports Pain Patches Manufacturer or OEM, and thoroughly vetting your Sports Pain Patches Supplier, you position your Private Label Sports Pain Patches brand for long-term growth.
From formulation to packaging to labeling and marketing, compliance at every level helps you avoid costly recalls, legal trouble, and damaged brand reputation. In today’s competitive wellness market, compliance is more than a requirement—it’s a business strategy.
Related Questions and Brief Answers
Q1: Do I need FDA approval to sell Private Label Sports Pain Patches?
A1: Not if the product follows the FDA monograph. However, it must still be registered, listed, and manufactured according to FDA rules.
Q2: What is the most common compliance mistake Private Label brands make?
A2: Inaccurate labeling or making unapproved marketing claims.
Q3: Can a Sports Pain Patches OEM help ensure compliance?
A3: Yes, a reputable OEM will provide documentation, handle FDA registrations, and ensure GMP manufacturing.
Q4: What kind of testing should my patches go through?
A4: Stability, microbiological, adhesive performance, and ingredient verification testing.
Q5: How do I verify if my manufacturer is FDA-registered?
A5: Ask for their FDA Establishment Registration Number and verify on the FDA website.
By following these steps and partnering with the right Sports Pain Patches Manufacturer, your Private Label Sports Pain Patches can stand out not just in performance, but also in compliance and trustworthiness—key traits for any long-lasting consumer brand.